▶ Research
1. X-ray crystallography of drug target membrane protein
Membrane proteins play important roles in cell functions such as cell signaling, it fulfills approximately 30% of total proteins. Most of membrane proteins are target for drug development, more than 50% of commercialized drugs are known to act onto membrane proteins. The three-dimensional structure of membrane proteins is important for drug design, however, the three-dimensional analysis of membrane proteins is very difficult. The structure of only 700 types of membrane proteins (only 50 from 8000 human membrane proteins) are still known. The development of the following 3 technologies are important for the systemic and molar study of membrane proteins structure.
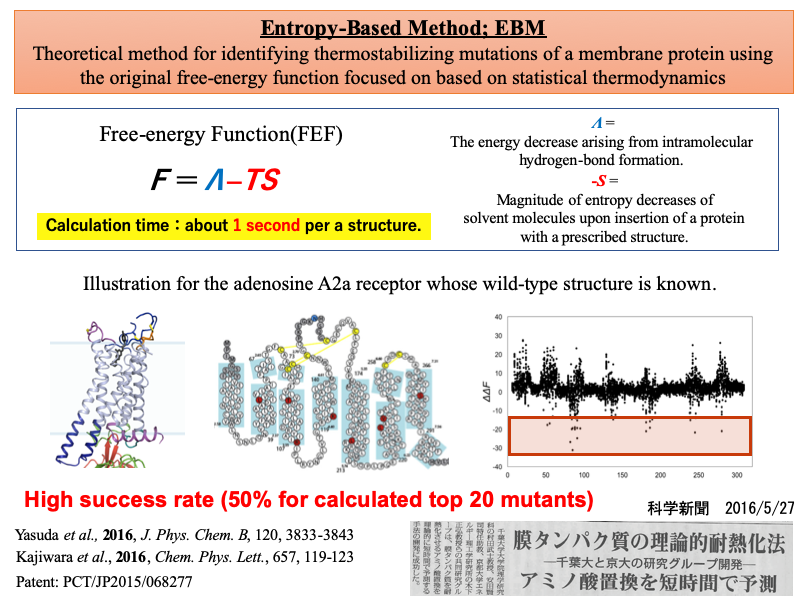
(1) Development of rational thermostabilization method for membrane protein based on statistical thermodynamics
(Collaboration with kinoshita lab., Kyoto University)
Structural determination of membrane proteins has been hindered by their inherent instability in detergent. Introduction of mutations can enhance their thermostability and stability in detergents, but the stabilizing mutations are currently identified by experiments. We develop a theoretical method for identification of thermostabilizing mutations that allows us to treat all of the possible mutations(Entropy Based Method; EBM). It employs a free-energy function that takes into account the translational entropy of hydrocarbon groups within the lipid bilayer as well as the protein intramolecular hydrogen bonding.
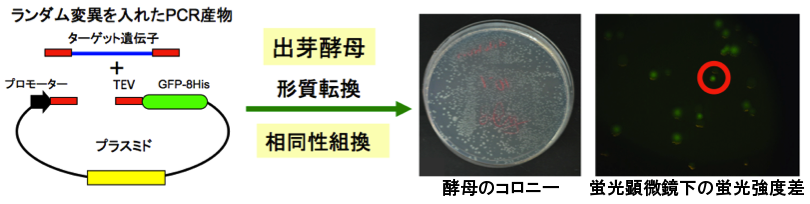
(2) Development of membrane proteins overexpression and purification system
Use Escherichia coli and Saccharomyces cerevisiae expression systems to screen the temperature stable membrane proteins mutants predicted by the above (1) theory. Moreover, we also develop a combination with random mutations using an evolutionary engineering approach that will provide a rapid screening for a more temperature stable and high expression protein mutants.
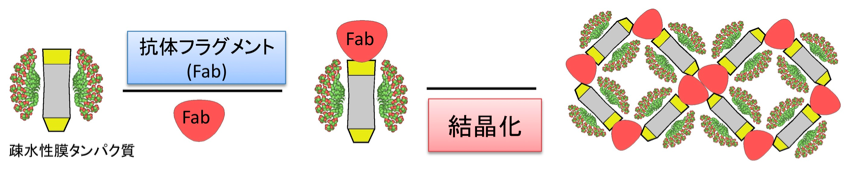
(3) Development of membrane protein crystallization method using antibody
One strategy to improve membrane protein crystallization is to extend the hydrophilic surface by the binding of an antibody that specifically binds the membrane protein. We are developing methods for immunization, screening for the production of monoclonal antibodies that bind to membrane proteins and membrane protein co-crystallization methods.
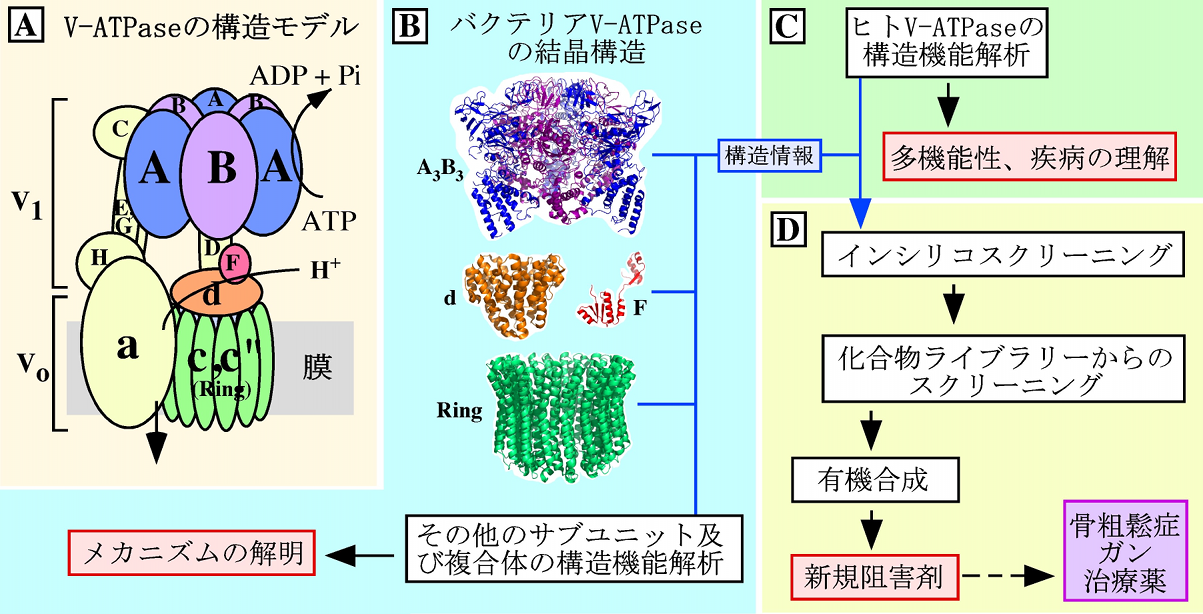
2. Structural/Functional analysis of V-ATPase toward drug development
Vacuolar ATPases (V-ATPases) function as proton pumps in acidic organelles and in plasma membranes of eukaryotic cells. V-ATPases are expressed also in osteoclasts and cancer cells membranes, and are known to be involved in diseases such as osteoporosis and cancer cell proliferation and metastasis. The understanding of V-ATPase molecular mechanism and the search for V-ATPase inhibitors is important for the development of therapeutic drugs for these diseases.
We discovered a bacterial V-ATPase homologous enzyme. To clarify the molecular mechanism of V-ATPase we have analyzed the structure and function of this enzyme. In addition, we are using the methods developed with bacteria V-ATPase studies to analyze the structure and function of human V-ATPase. Currently, we are screening chemical compound libraries to search for V-ATPase inhibitors, and develop a new inhibitor based on the structure of the obtained compound.